
Webinar
Selling pharmaceuticals to the government: the 65IB federal supply
In the first of a two-part webinar series hosted by the Coalition for Government Procurement (CGP), Baker Tilly discussed common challenges faced pharmaceutical manufacturers selling to the federal government through the Federal Supply Schedules program.
This webinar armed attendees with information on how to make a positive impact under these agreements, and included a discussion of best practices for contract negotiations and administration. Life Sciences government contractor advisors at Baker Tilly also explored FSS statutory and compliance requirements.
Highlights included
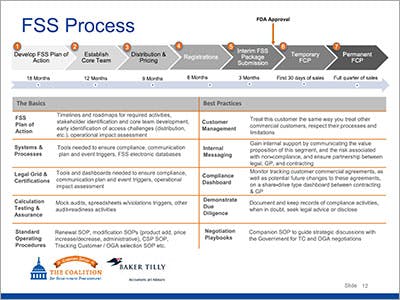
- Drug Launch Considerations
- Negotiation Strategies
- Best Practices for Operationalizing a FSS Contract
- Managing your Customer
Download the presentation:
